Today’s Wonder of the Day was inspired by Andrew from San Diego, CA. Andrew Wonders, “What are nutrons, electrons, and protons?” Thanks for WONDERing with us, Andrew!
Do you ever look at the world around you and WONDER about what things are made of? As you begin to observe things more closely, you realize there are many levels at which you can examine things.
For example, consider the tasty campfire treat we call the s'more. What's it made of? That's easy! It's made of chocolate, marshmallow, and graham cracker. You can dig deeper, though.
What is the chocolate made of? What's a marshmallow anyway? How were the graham crackers made? If you look at the list of ingredients on a chocolate bar, a package of marshmallows, and a box of graham crackers, you'll realize there's much more than meets the eye.
Scientists learned long ago that that's pretty much true about everything in the world around us: there's much more than meets the eye. In fact, the tiniest building blocks of matter — called atoms — can only be "seen" with powerful electron microscopes.
An atom is the smallest particle of an element that can exist on its own. In other words, if you have a bar of solid gold, the smallest particle that could exist by itself and still be considered gold would be one atom of gold.
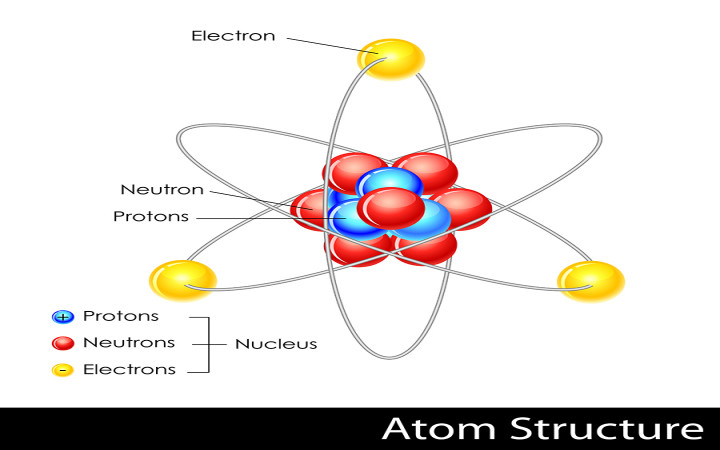
Atoms are composed of three primary particles: protons, neutrons, and electrons. Neutrons and protons together make up the dense center of an atom, known as the nucleus. Electrons orbit in shells in the space around the nucleus.
Neutrons have no charge, while electrons have a negative charge and protons have a positive charge. These charges are responsible for the electromagnetic force that keeps the electrons orbiting around the nucleus.
Atoms are curious particles when you think about them. Almost all of an atom's mass comes from the protons and neutrons in the nucleus. However, because electrons orbit around the nucleus, most of an atom is empty space! The nucleus only accounts for approximately 1/10,000th of the size of an atom.
You can't see atoms with the naked eye, because they're simply too small. Using electron microscopes, scientists have been able to study atoms.
An atom measures approximately 1/10th of a nanometer in diameter. That's about 100,000 times thinner than the average strand of human hair. If you made a straight line of 43 million iron atoms side by side, the line would only measure one millimeter long.
If all matter is made up of atoms, how do we get different elements? It all depends upon the internal structure of the atoms. Each element has atoms with a unique structure and a different number of protons. For example, each atom of hydrogen has one proton, while each atom of carbon has six protons.
Some elements even have more than one form. The other forms, called isotopes, have the same number of protons but a different number of neutrons. For example, hydrogen, which normally has no neutrons, has two isotopes: deuterium with one neutron and tritium with two neutrons.