Today’s Wonder of the Day was inspired by Josie. Josie Wonders, “How does hydrogen peroxide work?” Thanks for WONDERing with us, Josie!
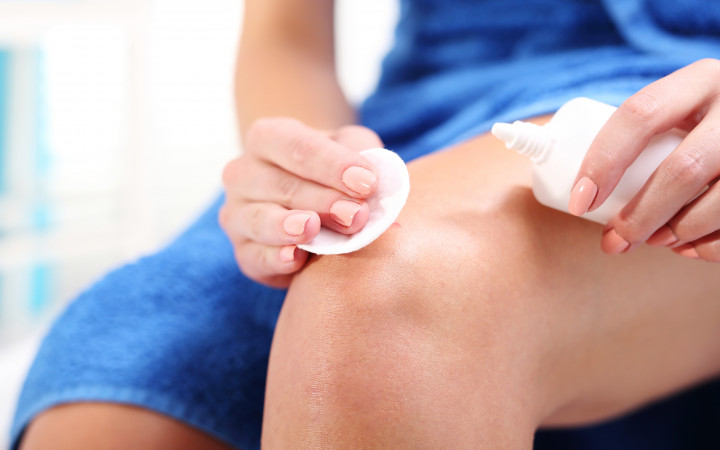
Have you ever tried to ride a skateboard? The thrill of speeding down the sidewalk can be …as long as you stay on your board. Unfortunately, science often intervenes in the form of friction and gravity, leading to scraped knees and elbows.
If you've ever gotten a cut or scrape, you may have had a friend or family member grab a brown plastic bottle of liquid that looks like water. When they poured it over your scrape, it fizzed and stung a little bit. What was it and why did it do that?
The clear liquid in the brown plastic bottle is hydrogen peroxide. Specifically, it is a 3% solution of hydrogen peroxide, which means it consists of 97% water and 3% hydrogen peroxide.
Hydrogen peroxide is a compound made up of two hydrogen atoms and two oxygen atoms. Scientists write the formula for hydrogen peroxide like this: H2O2.
At room temperature, hydrogen peroxide is stable. It won't foam inside the bottle or on healthy skin. It is sensitive to light, though, so that's why it comes in a brown plastic bottle and needs to be stored away from sunlight.
When poured onto a cut or scrape, hydrogen peroxide encounters blood and damaged skin cells. These contain an enzyme called catalase, which breaks down the hydrogen peroxide into water and oxygen.
The fizzing you see in the form of bubbles is the oxygen gas escaping. Catalase can cause up to 200,000 reactions per second. This powerful foaming action can help clean dirt, dried blood, and damaged cells out of a wound.
Hydrogen peroxide also kills certain types of bacteria. Some bacteria contain catalase, which helps to defend the bacteria from hydrogen peroxide. Other types of bacteria, however, can be killed by hydrogen peroxide when it destroys their cell walls.
Hydrogen peroxide also has bacteriostatic properties. That means it can keep bacteria from reproducing and spreading, thereby reducing the chance of an infection growing. It also can act as a sporicide, killing fungal spores that can cause infection.
Despite these benefits of hydrogen peroxide, most dermatologists don't recommend using it to disinfect open wounds. Why? It can also kill healthy cells alongside bacteria and fungal spores. In particular, it can kill fibroblasts, which are a special connective tissue the body uses to repair wounds.
In some cases, using hydrogen peroxide to disinfect wounds can slow the healing process because of the fibroblasts it kills. If it kills too many healthy cells around the edge of a wound, hydrogen peroxide can also worsen scarring.